Abstract
Clonal hematopoiesis of indeterminate potential (CHIP) is a sine qua non-phenomenon of aging. However, clonal hematopoiesis is also common after chemotherapy treatment and is closely related to the development of therapy-related MDS/AML. While clonal hematopoiesis has been investigated in a variety of contexts, it is understudied in Hodgkin's lymphoma (HL). Hodgkin's lymphoma is a unique B-cell neoplasm with an exuberant and diverse microenvironment that exceedingly outnumbers the malignant cell of interest (Reed Stenberg cells). There is recent evidence about clonal hematopoiesis in the tissue microenvironment of HL as well as in the non-neoplastic cells. In this study, we set to explore the distribution of somatic mutations in the non-neoplastic cells of HL patients and hypothesized if the presence of specific mutations in the mononuclear cells would yield a better response to immune checkpoint inhibitors (ICI).
We took advantage of the bio-banked peripheral mononuclear cells obtained from predominantly relapsed/refractory HL patients treated with immune checkpoint inhibitors (ICI) at our center. We analyzed the distribution of somatic mutations using deep targeted sequencing using a commercial panel [NovaSeq 6000, PE 150]. The effective average sequencing depth for the sample was 1,000X. Variants calling and data interpretation in NovoPM were done using Novogene's bioinformatics pipeline.
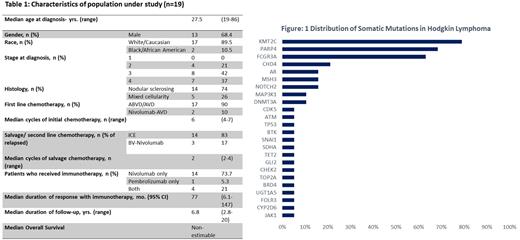
Peripheral mononuclear cell samples obtained from 19 HL patients treated at our center with ICI (14 nivolumab only, 1 pembrolizumab only, and 4 both) were sequenced. The median age at diagnosis was 27.5 years. and 68.4% were male. Most patients (79%) were diagnosed with stage III or IV disease, and 74% had nodular sclerosing histology. Most patients in the cohort received ICI in the salvage setting only (4 in the second line and 13 in subsequent lines). Patients received a median of 2 lines of therapy prior to ICI (range 0-5). The median duration of follow-up was 6.8 years (2.8-20). The median duration of response to ICI was 77 mo. (95% CI 6.1-147 mo.). The best response of ICI was CR in 7, PR in 11, and stable disease in 1 patient. The mutation testing in all patients was performed after exposure to prior chemotherapy and immunotherapy. All patients (100%) were found with at least had one mutation, with KMT2C being the most common (79%), followed by PARP4 (68%), FCGR3A (63%), CHD4 (21%), AR (16%), MSH3 (16%), NOTCH2 (16%), MAP3K1 (11%), DNMT3A (11%) and TET2 (5%). Median VAF for the most common mutation KMT2C was 17.5% (8.8 to 24.6), and PARP4 was 9.6% (6.7 to 16.3). The median number of mutations per patient was 4 (1-8). On comparing patients with <4 vs. 4 or more mutations, there was no difference in the median duration of response to ICI (non-estimable in both groups, p 0.4). 15 of the 19 patients (79%) had in-dels, with HNF1A and BCR being the most common (67%). Our cohort had no increased incidence of MDS/AML until the most recent follow-up.
We show that KMT2C is a recurrently mutated gene in samples of chemotherapy-treated patients for HL and represents clonal hematopoiesis. Prior studies have demonstrated that KMT2C deletions enhance hematopoietic stem cell self-renewal and do give a fitness advantage in the presence of chemotherapy. None of the patients in our cohort has developed therapy-related MDS/AML though it could be a risk in the future.
Disclosures
Balasubramanian:Kura Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal